Pressure-Temperature
YouTube ... Quora ...Google search ...Google News ...Bing News
Contents
Temperatures
The temperature at which a compound vaporizes is called its boiling point. The boiling points of different compounds vary, so by controlling the temperature, the desired compounds can be separated from the plant material.
For example, to distill THC, the temperature would be set to 157°C (315°F). This would cause the THC to vaporize and rise up the distillation column, where it would be condensed back into a liquid. The other cannabinoids and terpenes would remain in the plant material.
The temperature at which a compound is distilled can also affect its purity. If the temperature is too high, the compound can be degraded. If the temperature is too low, the compound may not be completely vaporized. It is important to use a precise temperature control system when distilling cannabis. This will help to ensure that the desired compounds are separated from the plant material and that they are distilled at the correct temperature to maintain their purity.
- Cannabinoids:
- THC: 157°C (315°F)
- CBD: 180°C (356°F)
- CBG: 126°C (259°F)
- Terpenes:
- Limonene: 177°C (350°F)
- Linalool: 198°C (388°F)
- Pinene: 156°C (312°F)
- Solvents:
- Ethanol: 78.5°C (173.3°F)
- Butane: −0.5°C (31.1°F)
Short Path Distillation
Short Path Distillation: is a technique used to separate and purify different compounds in cannabis oil, such as cannabinoids and terpenes. The process involves heating the cannabis extract under vacuum conditions to reduce the boiling points of the desired compounds. The following are approximate temperature ranges used in Short Path Distillation:
- Heads Fraction: The heads fraction typically contains volatile compounds with lower boiling points, such as terpenes. The temperature range for collecting the heads fraction is around 248 to 302 degrees Fahrenheit (120 to 150 degrees Celsius).
- Body Fraction: The body fraction consists of the desired cannabinoids, including THC and CBD. The temperature range for collecting the body fraction is approximately 302 to 392 degrees Fahrenheit (150 to 200 degrees Celsius).
- Tail Fraction: The tail fraction usually contains heavier compounds and impurities. The temperature range for collecting the tail fraction is around 392 to 572 degrees Fahrenheit (200 to 300 degrees Celsius).
Crystallization
In certain cases, a final step is taken to separate THC from CBD. Crystallization is a common method. A reactor vessel is filled with feedstock and a solvent which is chilled slowly from 60 degrees Celsius to minus-20 degrees Celsius. A slurry results and that is transferred to a Nutsche filter dryer to produce pure, dried crystals. The Nutsche filter is a jacketed vessel in which the temperature is controlled with a circulating hot oil unit. The process results in a 98% or higher purity of the CBD or THC product.
Pressure-Temperature
- Boiling Point of THC - Bleaker ... nomagraph and spreadsheet below
- Raoult's Law and Ideal Mixtures of Liquids | Chemistry LibreTexts
- Vapour Pressure-Composition Diagram for Ideal Solutions | Minia
- 8.9: Distillation - Chemistry LibreTexts
- 4.5: Separating Volatile Solutions - Chemistry LibreTexts
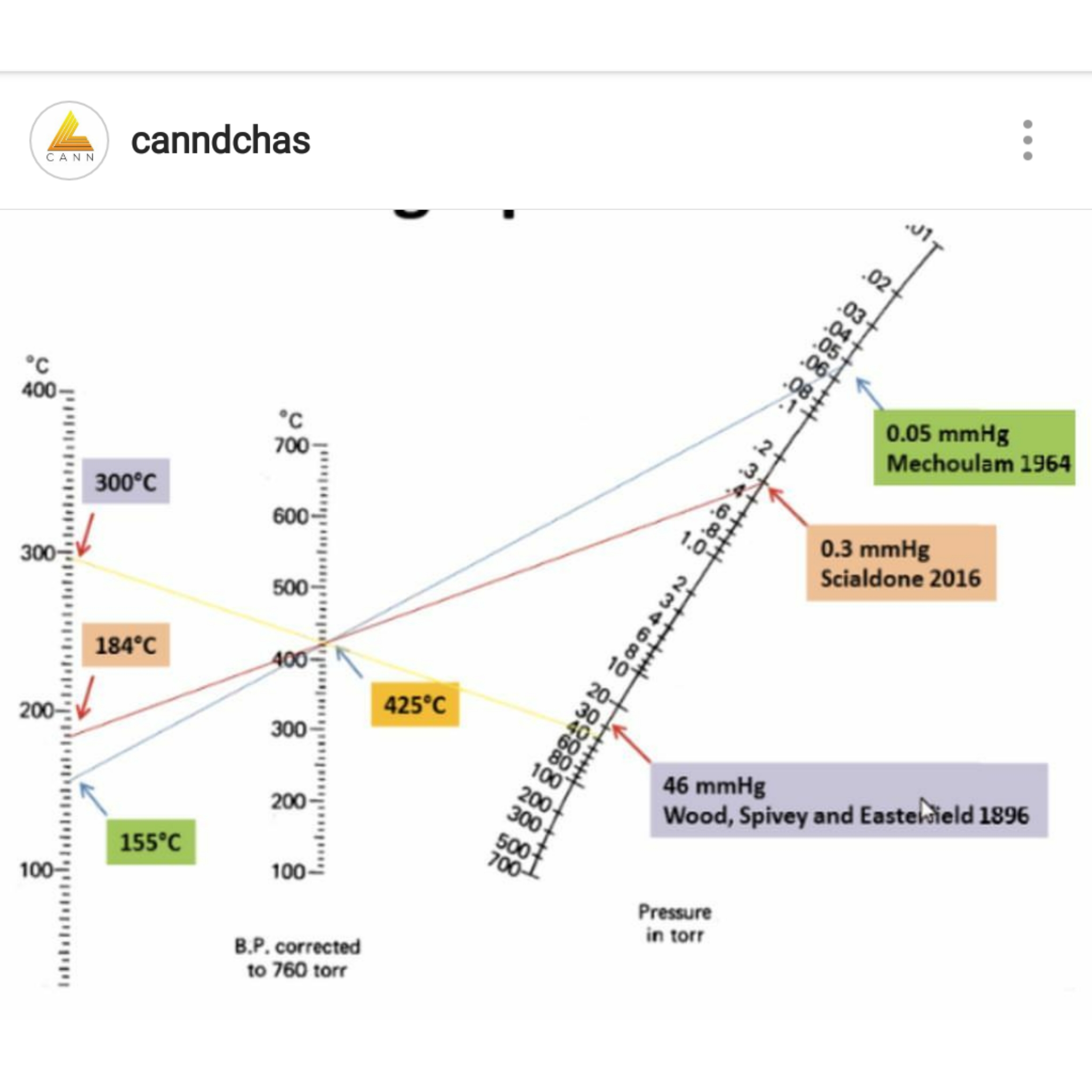
Nomograph: You find those numbers on the lines and draw a straight line between them. Where that line crosses the other line is the answer to your problem.
The chart (and the math that created it) was the basis for the computation for the pressure curve really I created. If you observe two known points of a boil at two different pressures then the math that created that curve chart you posted can be used to extrapolate all the rest of the points along the curve. The chart you posted uses mmHg for the pressure unit; .01 mmHg listed as the top pressure value = ten microns. I use microns as a unit of reference. The math gives crude estimates.
If you take the two known points I used you can plot it on that chart. “Boiling” is somewhat a subjective term at these pressures. I used the bottom of the bottom of the cold finger glass of the sublimation apparatus to judge when accumulation became noticeable as the “boiling” point. The two data points used in the calculation for the D9 THC chart were 120C at ¾ of one micron, and 200C at 20 microns. You can plug these points into the chart above and see how high the boil would be at room pressure which is 760,000 microns (the middle of your chart is corrected to 760 Torr = 760,000 microns). A theoretical perfect vacuum is zero microns.
From that chart you posted it is also possible mathematically to compute the enthralpy of evaporation. This number means very little to the daily refinement but it does offer clues about what is going on inside the rig when it runs and it helped me to figure out my horizontal distillation set up. I run the distillation pass horizontally via kugelrohr bulbs but use a heating mantle and stir bar instead of a formal oven. The temps run much lower to pull across distillate this way and the high numbers suggested this would work better than a traditional vertical distillation. It does. It is because you are not sinking all that heat into the uptake path in a horizontal mode.
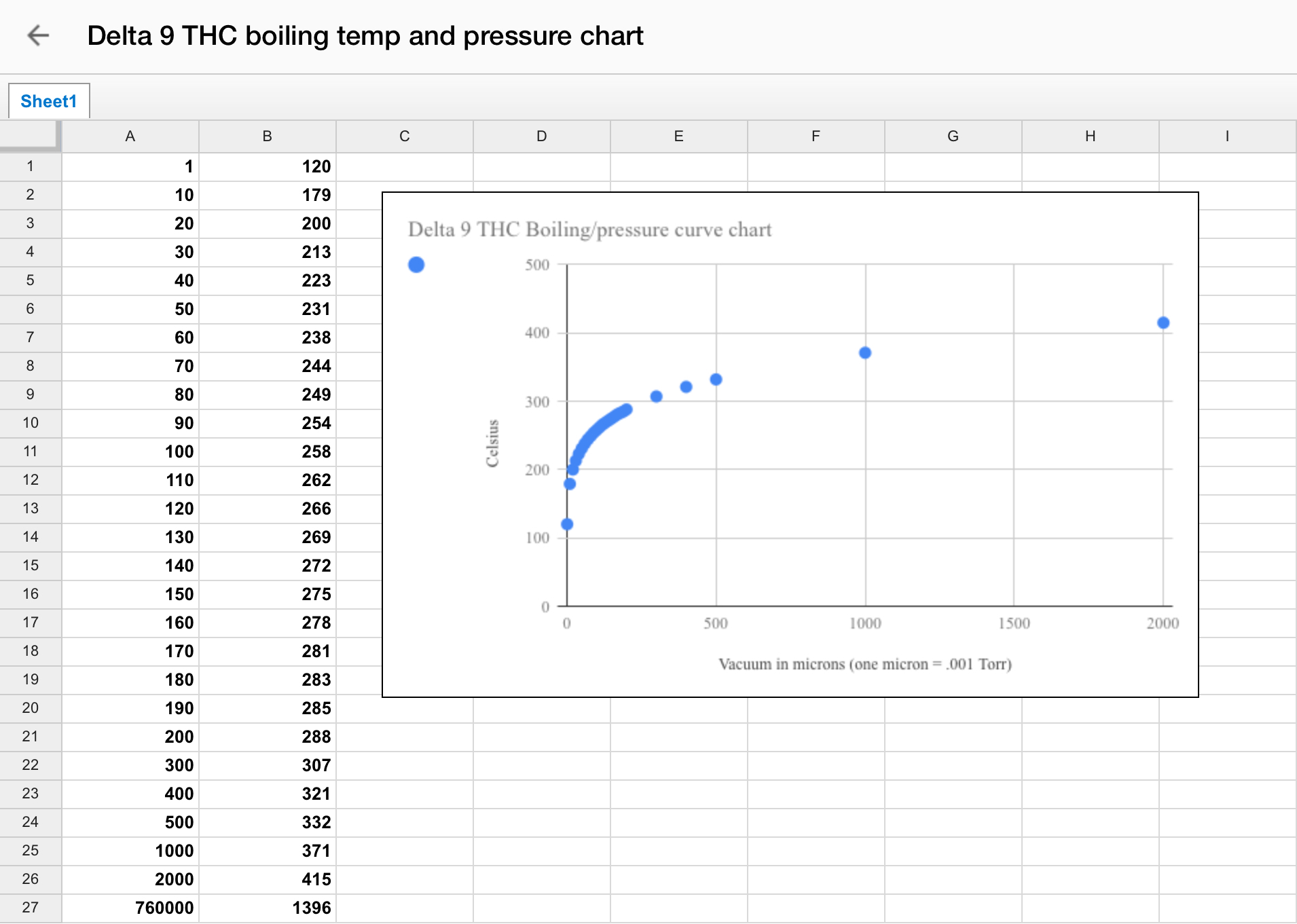
Pressure-Temperature Nomograph
To fully understand distillation, one must consider the vapor pressure vs. composition plots for a hypothetical mixture at some arbitrary temperature at which both liquid and gas phases can exist, depending on the total pressure. You can use tools such as the Pressure-Temperature Nomograph Interactive Tool to calculate a boiling point or pressure using the Antoine Equation. You will need to adjust the temperature and vacuum settings according to the boiling points of different cannabinoids. For example, THC boils at around 157°C (315°F), while CBD boils at around 180°C (356°F).
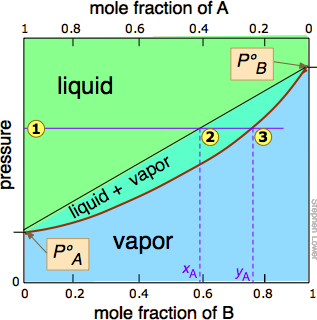
Vapor pressure vs. composition plots are used to understand the behavior of mixtures of two volatile liquids. These plots show the relationship between the vapor pressure and the composition of a mixture at a given temperature. The vapor pressure of a mixture is determined by the vapor pressures of its individual components and their mole fractions in the mixture. Raoult's Law states that the partial vapor pressure of each component in an ideal mixture is equal to the product of its mole fraction and its vapor pressure as a pure substance.
In an ideal mixture, the intermolecular forces between two molecules of one component must be exactly the same as the intermolecular forces between a molecule of one component and a molecule of the other component. This is why mixtures like hexane and heptane get close to ideal behavior. They are similarly sized molecules and so have similarly sized van der Waals attractions between them.
A vapor pressure vs. composition plot can be constructed by plotting the total vapor pressure of the mixture against the mole fraction of one of its components. The plot will show two curves: one for the liquid phase and one for the vapor phase. The liquid composition curve lies above the vapor composition curve. The intersection of a horizontal line representing the total pressure with these two curves defines the compositions of the liquid and vapor phases in equilibrium at that pressure.
Vapor pressure composition plot vs Pressure-temperature chart
A vapor pressure composition plot is a graph that shows the relationship between the vapor pressure and the composition of a mixture at a given temperature¹. It is used to understand the behavior of mixtures of two volatile liquids. The plot will show two curves: one for the liquid phase and one for the vapor phase. The liquid composition curve lies above the vapor composition curve⁵.
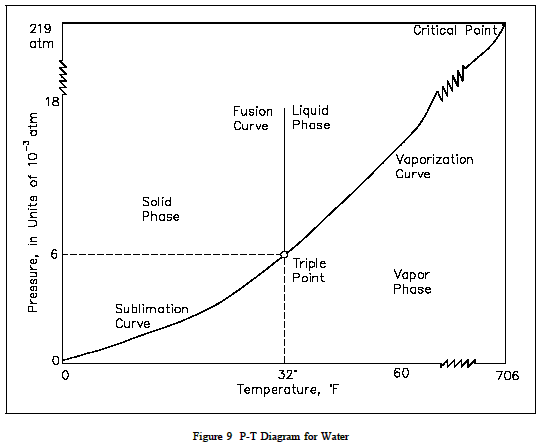
On the other hand, a pressure-temperature chart is a graph that shows the relationship between the pressure and temperature of a substance. It is used to understand how the pressure of a substance changes with temperature. For example, a pressure-temperature chart for water would show how the vapor pressure of water changes with temperature².
The main difference between these two types of graphs is that a vapor pressure composition plot shows how the vapor pressure of a mixture changes with its composition, while a pressure-temperature chart shows how the pressure of a substance changes with temperature.
Molecular Weight - mole
- Molecular Weight: Molecular weight is the mass of a molecule that is calculated by adding the atomic weights of its constituent atoms. It can be expressed in terms of atomic mass units (amu) or Daltons (Da). Molecular weight is used in chemistry to determine stoichiometry in chemical reactions and equations.
- Mole: is a unit of measurement used in chemistry to measure the amount of a substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. It is similar to other units of measurement like a dozen or a pair, but instead of representing 12 or 2 items, one mole represents 6.02214076 × 10^23 particles. This number is known as Avogadro's number and is used to convert between the number of atoms or molecules and the mass in grams. The mole is used as a unit of measurement because it provides a consistent way to convert between the number of atoms or molecules and the mass in grams. This makes it easier to perform calculations and understand the amounts of substances involved in chemical reactions.
In a vapor pressure vs. composition plot, the mole is used to represent the number of particles in a mixture. The plot above shows the relationship between the vapor pressure and the composition of a mixture at a given temperature. The vapor pressure of a mixture is determined by the vapor pressures of its individual components and their mole fractions in the mixture. A mole fraction is the ratio of the number of moles of one component to the total number of moles in the mixture.
Short Path Distillation of Cannabis THC & CBD (Tetrahydrocannabinol & Cannabidiol)
yeah okay so we're gonna start with a hundred and eighty-three grams at this starting material here CBD 60 percent award-winning sour tsunami sour tsunami CVD we're gonna run it through the short path well chilled magnetic concentrator the month we're here with the oil tycoon concentrates pellets and we're gonna be run into the material here got the welch we're cranking over here the Dewar condenser chilled now it's time to load so I'll show you guys how we load the system we got the flask wand up to about a hundred degrees pullover how many of these Celsius is to keep things warm there's a qat of the moisture inside the long glass or anything here is our 24 degrees Celsius it's a vapor temperature in the head here we've got water running the reason we're getting some air bubbles and the condenser is we've got up missing a seal on this bottom GL connection so it's adding a little bit of oxygen into there but it's still working properly and then over here we've got the chiller warming up booze out 40 degrees Celsius and it's recirculating back in the back here we've got it connected with just some quick water lines quick so let's go ahead load the system and begin check out what I'm doing so we're just gonna fold this patty up they almost leave your hand there just that like just for reference so this is we're gonna run 183 grams watch okay so here we are just loading up quite a bit in through the metal funnel here just adding it right into the side for you could see pulling a vacuum to us assist pulling through here you're loading probably about 200 grams ish we're there a little bit more here 60% CVD 3.9 percent THC in there concentrate there we go I'll keep you guys sooner in here as we continue this process is it agitating yeah all right you guys we're back we're just starting to reach our first fraction so I figured I break the camera out and show you guys where we're at we're actually at a hundred and ninety two degrees Celsius and we're really climbing through this area however we'll reflux them pretty good this is where we're gonna start refluxing in the head here in the vigor of and essentially start to see some of your terpenes and other more volatile compounds that's our temperatures here so here you can see the consistent differet that we're achieving 192 degrees Celsius is your friend except Amana 125 degrees Celsius at the head and 125 here at 127 is climate as the thermo probe of the head here Steve now the reflux is kind of started to slow down a they grow a little bit so you can tell we're just kind of trying to reach through destruction and as soon as we get to about 2 32 to 40 we should start to see to pick up speed and really get thicker and towards also collecting a little bit absolute herbs you can see now we are getting cold traps across here in trips in the col.caf across the line things that are just not necessarily condensing in the head and are making your way of past that's allow presentation before the vacuum pumps or bastard we've got we've had the same amount there faster than we did last time you have a high volume get like what three times the volume and yeah so reaching to around six degrees Celsius and climbing so we'll catch back with you guys here in about 25 degrees alright you guys we are back I figured I'd just check in with you we broke past 220 which we we're stuck out for a little while just kind of fractioning off the terpenes which we've now rotated the flask you can see here this is where we've collected all that quite viscous I move it around you can kind of see just a little jiggle and then over onto this glass we've started to thicken up and you can just see it now dripping on in this is a CVD prominent material that we are running today this our tzunami oil tycoon and you can see the color on this middle class is just epic so we'll continue to fraction off here where again 224 Celsius on the mantle set points to 35 on the mantle by the set point is 235 you can see in the vigor oh the color in here is just like water in it so we'll wait till we get to about 235 things should thicken up a little darker in color you would expect a really uh fraction oats off some of the probably the head stash of the run here you would say now you can see the consistency what we're saying get in there get something right now that we're dripping on access window there you go look at that little nugget it's falling out look at that you guys the color on this is epic so this is the open source steel 5 litre head running on a 2 liter flask here at 2 meter manifold and the 2 liter Mansel collection Mantle's our collection class we use index around bottom for 250 milliliters to these are 250 mils on the collection flask here and then up top we've got the thermo scientific thermocouple in there we've just got a little PTFE tape to make the diameter the width that will secure down in one of these thermal adapters and then over here we've got a thermocouple that's connected connected to the mantle reading the internal temperature of the boiling back here so we'll catch back with you about 10 degrees or so all right you guys we're back I want to show you the progress that we've made here on our fractions we're getting down here these are darkening up consistently that's the molar concentration of black is remaining and the lighter pistons these are being taken out you can see here our separate fractions try to get it a different angle for you guys so down below we've got our collection flask here the 250 ml which is our most recent attraction and next up here we have a nice color and consistency in this wall here and then a final up here would be more of our terpenes and lower boiling point items here so things are coming along pretty good here we're writing down all the data and the variables throughout the head the man told a set point and remaining a fraction at 2:32 this has been a lower fraction than some of our recent runs but we're getting pretty good flow so we figured we kind of just stick here and also the temperature has been fighting considering we're getting a good amount of condensation up in the condenser here one thing to note too James is that this bottom flask is turning quite green that's probably the corridor we're down here at the very bottom canonically yeah yeah we're gonna watch and keep an eye on the color and consistency and see if we don't see some lipids and some cloudiness starting to form up on the backend of the count here yeah you can usually see it when you're looking at the bottom of the of the putter here they're receiving head you can see that it spreads out you can start to see the cloudiness form if you if you watch here I mean obviously you can watch it inside the dirt inside in the bottom class but if you watch them spreading out of the year and receiving [Music] we've got a pretty good just written out it's not the street at all right now like it has been so so further than finishing off this last fracture today moving into the next day there we go starting to flow up pretty good [Music] we'll just keep an eye on color and consistency from here on out raise the mantel temperature just a little bit but we are getting towards the bottom we don't want to run this glass dry so keep your eye on things and we'll update you guys as we finalize up yeah one thing to know to quickly put in is that this this reflection here is slowed down quite a bit it was doing it a bunch up in here him and the bumping was getting him up almost up on her shoulder I mean it's pretty good they were running this five layer head on this two liter flask I mean we've got quite a bit of bigger indentations on this one compared to the two liter and we're getting significant bumping up into the head here they're right high bar my everything looks quite a bit past the point almost up to the shoulder you know it was just enough hype there in the column to restrict that from happening and hopping over but things are looking good so stay tuned you guys and we'll finalize out this short past session key to get stated they're not much every but you guys were back here finalizing it the final fraction here did you use people and you can see how a green and nasty and dark it is we obviously don't want to be fracturing off this no longer are we getting any reflux in the bigger up column and on the tail end here we're getting a nasty cloudiness that we don't really want to be adding obviously probably adding a pretty bad visual appeal to people who might want to be using it but then again might be some compounds you don't necessarily want to be having in there in the first place so we'll probably take this ball clean this out here add some more in and just refraction and then that'd be the short path here's the middle Dragon Ball which comes out with your top shelf fine eighth grade very nice color and consistency on this here and then here is your first fraction of the terpenes with a little bit of yellow drippin Furness THC this one is a little clear in the middle but if you read refraction it put it through a second pass and sure you get well over 90% THD for sure or CVD in this case go up to wait till the tests come back on the concentrations of the compounds in there but we'll keep you guys posted hopefully enjoyed this session at short path distillation using the open source steel here hopefully you enjoyed it that's the five layer head on the two liter flask rock on the three 250 ml dragonballs on it calories fever here with the inline cold trap and over here off to the side the welch 1400 duo seal and last but not least the VW are recirculating back so we're gonna turn the head temp up to finalize things out so we're going to turn it up to about 70 degrees Celsius that'll help warm this condenser up to get this stuff to flow out of here and drip it'll get stuck here right here at the end because there's obviously no condenser we'll take it off heat it up and just drip it back into one of the dragonballs being this one here but there you go there's the finalize process hopefully enjoy so next time